2022年论文
2022年论文
1. Wang, X.-K.; Jia, Y.-M.; Li, Y.-X.; Yu, C.-Y.,* Total Synthesis of Pseudouridimycin. Org. Lett. 2022.24 (2), 511-515.
2. Li, Y.-X.; Wang, J.-Z.; Shimadate, Y.; Kise, M.; Kato, A.; Jia, Y.-M.; Fleet, G. W. J.; Yu, C.-Y., *Diastereoselective Synthesis, Glycosidase Inhibition, and Docking Study of C-7-Fluorinated Casuarine and Australine Derivatives. J. Org. Chem. 2022.87 (11), 7291-7307.
Abstract
C-7-fluorinated derivatives of two important polyhydroxylated pyrrolizidines, casuarine and australine, were synthesized with organocatalytic stereoselective α-fluorination of aldehydes as the key step. The strategy is extensively applicable to some synthetically challenging fluorinated iminosugars and carbohydrates. The docking studies indicated that the potent inhibitions of trehalase and amyloglucosidase by the fluorinated polyhydroxylated pyrrolizidines are due to the interaction modes dominated by fluorine atoms in these iminosugars with the amino acids' residues of the corresponding enzymes. Steady interactions were established between the C-7 fluoride and a hydrophobic pocket in amyloglucosidase by untypical anion−π interactions. These unexpected docking modes and related structure–activity relationship studies emphasize the value of fluorination in the design of polyhydroxylated pyrrolizidine glycosidase inhibitors.

3. Wang, J.-Z.; Cheng, B.; Kato, A.; Kise, M.; Shimadate, Y.; Jia, Y.-M.; Li, Y.-X.; Fleet, G. W. J.; Yu, C.-Y., *Design, synthesis and glycosidase inhibition of C-4 branched LAB and DAB derivatives. Eur. J. Med. Chem. 2022, 233, 114230.
Abstract
Two series of C-4 alkylated and arylated LAB (1,4-dideoxy-1,4-imino-L-arabinitol) and DAB (1,4-dideoxy1,4-imino-D-arabinitol) derivatives, synthesized in 6 steps from enantiomeric cyclic nitrones derived from L- and D-tartaric acid, were designed and assayed against various glycosidases. C-4 Branched LAB alkyl and phenyl derivatives 5La-d showed potent a-glucosidase inhibition, particularly against human lysosomal acid a-glucosidase; C-4 DAB derivatives 5Da-d, with small alkyl groups, showed enhanced inhibition of rat intestinal maltase and sucrase. Both enantiomeric C-4 arylated derivatives 5Lf-l and 5Dfl exhibited potent and selective a-glucosidase inhibition; and compound 5Li with a para-electron donating group (EDG) on its C-4 aryl group, showed the most potent rat intestinal sucrase inhibition. Docking studies showed similar hydrogen bonding modes for the iminosugar skeletons of DAB (1) and LAB (2) with ntMGAM,. While C-4 alkylated LAB derivatives showed high similarity in their binding modes with the active site of ntMGAM, binding modes of the DAB derivatives relied on the size of C-4 alkyl groups with methyl and butyl showed the optimum interactions. Furthermore, C-4 arylation improved the interactions of LAB derivatives with enzymes by T-shaped p-p stack with residue Trp-406; for C-4 arylated DAB derivatives, the p-p stack interactions were found with distinct planar distortions caused by EDGs or EWGs on the C-4 aryls. The results reported herein provided insights for the design and development of DAB and LAB related a-glucosidase inhibitors, and may also contribute to the future development of anti-viral, anti-diabetic and anti-Pompe disease drugs.

4. Kato, A.; Nakagome, I.; Kanekiyo, U.; Lu, T.-T.; Li, Y.-X.; Yoshimura, K.; Kishida, M.; Shinzawa, K.; Yoshida, T.; Tanaka, N.; Jia, Y.-M.; Nash, R. J.; Fleet, G. W. J.;Yu, C.-Y., * 5-C-Branched Deoxynojirimycin: Strategy for Designing a 1-Deoxynojirimycin-Based Pharmacological Chaperone with a Nanomolar Affinity for Pompe Disease. J. Med. Chem. 2022, 65, 2329-2341.
5. Wang, J.-Z.; Shimadate, Y.; Kise, M.; Kato, A.; Jia, Y.-M.; Li, Y.-X.; Fleet, G. W. J.; Yu, C.-Y., * trans, trans-2-C-Aryl-3,4-dihydroxypyrrolidines as potent and selective β-glucosidase inhibitors: Pharmacological chaperones for Gaucher disease. Eur. J. Med. Chem. 2022, 238,114499.
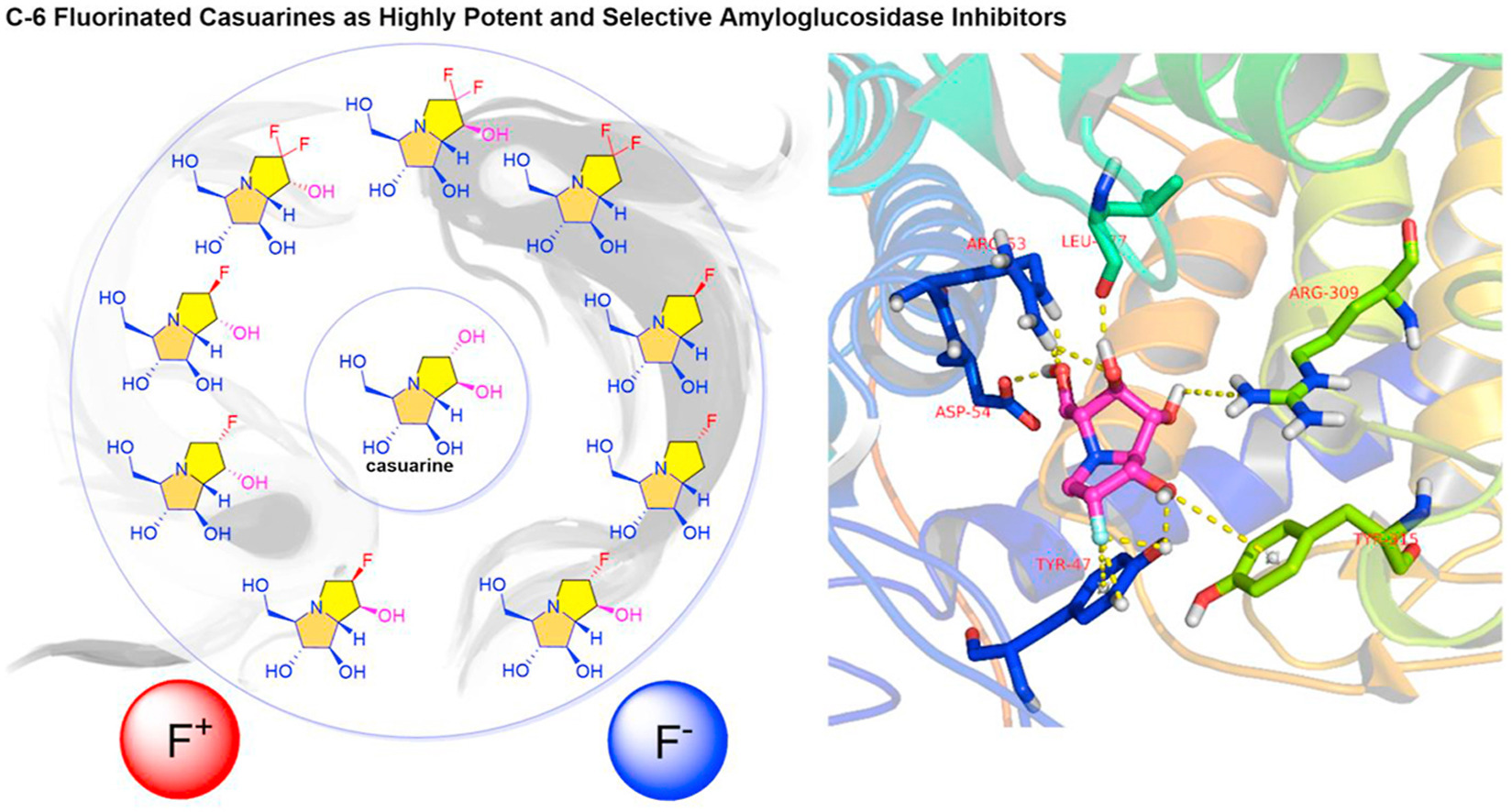
6. Li, Y.-X.; Wang, J.-Z.; Shimadate, Y.; Kise, M.; Kato, A.; Jia, Y.-M.; Fleet, G. W. J.; Yu, C.-Y. ,* C-6 fluorinated casuarines as highly potent and selective amyloglucosidase inhibitors: Synthesis and structure-activity relationship study. Eur. J. Med. Chem.2022, 244, 114852.
Abstract
A series of C-6 fluorinated casuarine derivatives have been synthesized via organocatalytic stereoselectiveα-fluorination of iminosugar-based aldehydes or direct nucleophilic fluorination of polyhydroxylated pyrrolizidines. Glycosidase assays against various glycosidases allowed systematic structure-activity relationship (SAR)study using molecular docking calculations. Introduction of fluorine atom(s) at C-6 position removed the trehalase and maltase inhibitory activities of all casuarine derivatives, and greatly increased their specificity towards amyloglucosidase. Inhibition of the fluorinated casuarines depended on the configuration of C-6 fluorine, of which 6-deoxy-6-epi-6-fluoro-casuarine (24) was found approximately 40-fold potent than its parent compound 6-epi-casuarine (2) as a potent and specific inhibitor of amyloglucosidase. Molecular docking calculationsshowed that replacement of the C-6 hydroxyls by fluorine atom(s) removed the original interactions with trehalase, but helped to reinforce the binding with amyloglucosidase via newly established fluorine related hydrogen bonding or untypical anion-π interactions. To further investigate the quantitative SARs of casuarinaderivatives, the CoMFA and CoMSIA models on amyloglucosidase were established, indicating the dominating effect of electrostatic field in amyloglucosidase inhibition. The 3D-QSAR models were validated to be reliable and can be used for further optimization of casuarine-related iminosugars, as well as design and development of anti-diabetic and immunomodulatory drugs.
7. Kato, A.; Nakagome, I.; Yoshimura, K.; Kanekiyo, U.; Kishida, M.; Shinzawa, K.; Lu, T.-T.; Li, Y.-X.; Nash, R. J.; Fleet, G. W. J.; Tanaka, N.; Yu, C.-Y. ,* Introduction of C-alkyl branches to l-iminosugars changes their active site binding orientation. Org. Biomol. Chem. 2022, 20, 7250.